Introduction
All-trans retinoic acid (ATRA) has been used with great success in acute promyelocytic leukemia (APL) cases with PML::RARA fusion gene (FG). There are still PML::RARA negative cases that manifest as APL, and they often carry FGs involved in RARA, RARB, or RARG. X::RARG positive APL ( RARG-APL) cases have been increasingly reported in recent years, thanks to the utilization of transcriptome sequencing (RNA-seq) and whole genome sequencing (WGS). Almost all evaluable RARG-APL cases were resistant to ATRA, and no mechanism has been elucidated. Intriguingly, cells transformed by artificial X::RARG were extremely sensitive to ATRA (PMID: 25510432), indicating intricate mechanisms unrevealed. This study first and systematically unveils the distinctive features and pivotal molecular mechanism of RARG-driven APL.
Methods
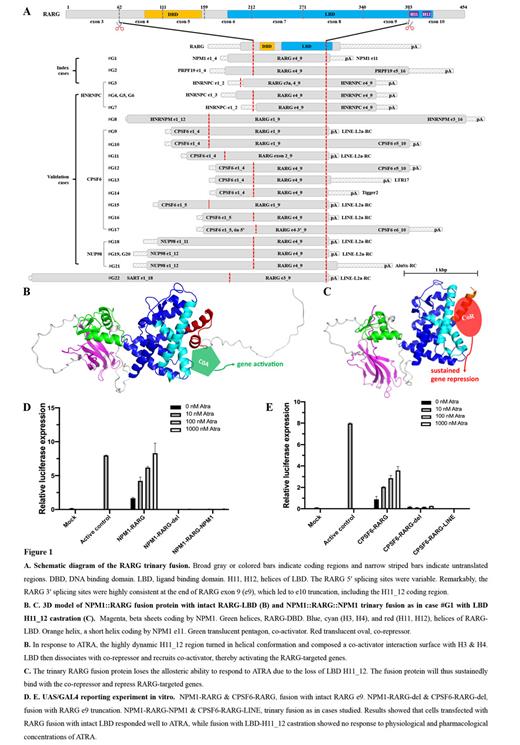
A total of 22 RARG-APL cases with RNA-seq and WGS data from 11 centers were enrolled (Fig. 1A). We used Arriba for routine FG calling and manually investigated the non-coding fusion sequence. Reverse transcription PCR (RT-PCR) and Sanger sequencing were used to validate the FGs and to determine whether the RARG 5′ and 3′ fusion events were located on the same cistron. The responsiveness of the fusion proteins to ATRA was evaluated using an optimized UAS/GAL4 reporter system.
Results
Among the cases enrolled, there were 15 males and 7 females, aged 0.9-69 (median 38) years. Morphological and immunophenotype analyses all showed features of APL. Routine FGs calling reported RARG fusion to a 5′ partner in each case, with variable RARG splicing sites (Fig. 1A). There were 7 RARG 5' partner genes, with CPSF6, HNRNPC, and NUP98 observed in multiple cases.
In the 3 index cases, we identified that they all had RARG 3‘ fusion events besides the 5‘ one (Fig. 1A). RT-PCR confirmed that the 5′ and 3′ RARG fusion events were in the same cistron in each case. Such as, the fusion transcript is the insertion of RARG-e4_9 (e refers to exon) between NPM1-e4 and e11 in case #G1, rather than two separate transcripts of NPM1-e4:: RARG-e4 and RARG-9:: NPM1-e11. We named this novel form of tandem splicingtrinary fusion.
Analysis of the 19 validation cases further confirmed that all RARG-APL cases had RARG 3′ fusion events (Fig. 1A). We also validated that the 5′ and 3′ RARG fusion events were in the same cistron in 3 more cases, of which archived cDNA were available. Remarkably, the 3' splicing sits of all cases were consistently at the end of RARG-e9, leading to RARG-e10 truncation. The RARG 3' partner gene was the same as its 5' partner in 11 cases. But in the other 11 cases, the RARG 3' partner was a transposon element (TE) sequence, the most common being a LINE-L2a (8 cases). Sequences analysis indicated that the committed locus of the involved LINE-L2a was at 4.2 Kbp upstream of the RARG gene. Gene expression analysis confirmed the aberrant activation of the involved TEs. All TEs involved in the RARG 3' fusions confer a poly_A signal sequence to the fusion transcript, which was essential for a mRNA. In-depth analysis indicated that TEs participate in the formation of RARG-FGs through a transposition mechanism rather than a translocation mechanism.
RARG-e10 encodes helix 11_12 (H11_12) of its ligand binding domain (LBD), which plays a pivotal role in allosteric response to ATRA (PMID: 31178221). The trinary fusion protein with RARG LBD-H11_12 castration will lose responsiveness to ATRA via an allosteric disability mechanism (Fig. 1B, 1C). Experiments confirmed that cells transfected with X::RARG fusion with intact LBD responded well to ATRA, while fusion with LBD-H11_12 castration showed no response to physiological and pharmacological concentrations of ATRA (Fig. 1D, 1E).
Conclusions
This study clearly revealed that trinary fusion and LBD castration are crucial molecular etiologies in RARG-APL. The trinary fusion orchestrates the aberrant activation of RARG (5‘ splicing) and the complete allosteric disability in responding to ATRA (3‘ splicing). Thus, this study elegantly explains the mechanism of leukemogenesis and the extensive ATRA resistance of RARG-APL. The formation of trinary fusion requires two steps of genome splicing or transposition events, which explains the rarity of RARG-APL. This also clarifies that RARG-APL has a significant disparity in molecular mechanism from PML::RARA-positive APL, and we suggest that it should be considered as a separate entity of acute myeloid leukemia.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal